Abstract
Background Severe SARS-CoV-2 infections associated with high mortality rates are reported in a higher percentage of patients (pts) with hematologic malignancies compared to the general population. In chronic myeloid leukemia (CML), pts with older age and uncontrolled disease have a higher mortality risk. The interaction between molecular response (MolResp) and other risk factors on mortality and severity in pts with SARS-CoV-2 infection has not been studied in detail so far.
Aims To identify the interaction between MolResp and other risk factors on mortality and severity in pts with SARS-CoV-2 infection.
Methods The iCMLf CANDID study collected data on SARS-CoV-2 positive CML pts from 157 institutions in 49 countries from March 2020 to November 2021 to determine the impact of patient, disease, and therapy specific factors on risk and outcome. Details on the registry were presented recently (Pagnano ASH 2021). 408 cases were added since the previous analysis. Severe disease was defined according to WHO criteria. Univariate and multivariate model analysis was performed using logistic regression.
Results Of 1050 pts, 676 (64%) pts were included in this analysis. Pts with non-COVID-19 related death, currently active COVID-19, lost to follow-up, post allogeneic transplant and pts with unavailable MolResp status were excluded. The median follow-up in this cohort was 2.1 (IQR: 1-3.8) months after COVID-19 onset. The mortality rate was 6% in evaluable pts. The average time to death post infection was 16 days. 23 (3%) pts had advanced disease (AP/BC) and 653 (97%) chronic phase (CP) at the time of infection. Of the CP pts, 519 (79%) pts had a MMR and 134 (21%) pts no MMR. Of pts not in MMR, 80 pts (60%) were not in CCyR. Mortality in patients in CCyR was 4%, compared to 13% for those not in CCyR. Line of TKI (p=0.89), gender (p=0.08) and duration of CML (p=0.6) had no effect on mortality. There was a higher proportion of pts with severe COVID-19 in pts with AP/BC (39%) or pts not in MMR (19% v pts with MMR 10%, p<0.0001).
The mortality rate for MMR, no MMR and AP/BC was 4%, 11% and 26% respectively. By multivariate analysis no MMR, AP/BC, lower country income, older age and coexisting comorbidities were independent risk factors for mortality. Using propensity score matching analysis, pts not in MMR had lower OS compared to pts in MMR (87% vs 97%, p<0.0001), supporting MolResp as an independent variable impacting mortality. Risk models based on multivariate logistic regression were developed to further investigate the interaction between these risk factors on mortality, and severity of SARS-CoV-2 infection. The mortality probability derived from the model ranged from 0 to 0.5 for CP pts. This involved 4 categories: low risk (LR <0.01), standard risk (SR 0.01-0.1), intermediate risk (IR 0.1-0.3), and high risk (HR >0.3). The mortality rate for LR was 0%, SR 4%, IR 15% and HR 40%. The severe disease rate for LR was 3%, SR 12%, IR 25% and HR 36%.
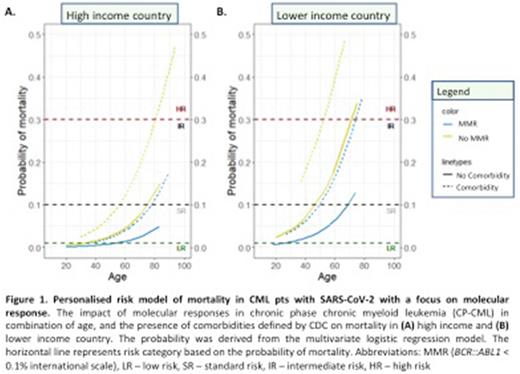
10% (n=14) of pts not in MMR were HR whereas 0.2% (n=1) pt in MMR was HR. In contrast, 4% (n=5) of pts not in MMR were LR while 28% (n=143) of pts in MMR were LR. Pts not in MMR had only 11% risk of death. The difference in mortality rates according to MolResp increased in older patients, and in pts with comorbidities (Fig 1A). MolResp had a larger impact on mortality in lower-income countries (Fig 1B).
Conclusion In comparison to pts in MMR, pts not in MMR had higher risk of mortality and severe disease. A similar trend was seen in patients according to CCyR status. Significantly, based on our personalised risk model, the risk of mortality, and disease severity was higher in patients not in MMR, and this is difference was greater in older patients, those with comorbidities and those from lower income countries. Pts categorized as CCyR, or not in CCyR had similar outcomes to patients categorized according to MMR status. This suggests that CML disease burden plays an important part in determining outcome with COVID-19, consistent with prior findings showing depth of MolResp correlated with immune status in CML (Hughes et al, Blood 2017). Notably the CANDID cohort had COVID-19 infections prior to the broad availability of vaccinations and antiviral therapies, and prior to the less severe omicron wave. Severity and mortality would likely be significantly lower for CML patients with COVID-19 in 2022. The impact of MolResp on COVID-19 outcomes in the current clinical setting warrants further investigation.
Disclosures
Chelysheva:Pfizer: Speakers Bureau; Novartis Pharma: Speakers Bureau. Cortes:Forma Therapuetic: Consultancy; Sun Pharma: Consultancy, Research Funding; Gilead: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Kartos: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Mauro:Sun Pharma/SPARC: Research Funding; AbbVie, Bristol Myers Squibb, Novartis, Pfizer, Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding. Milojkovic:Novartis: Honoraria; Incyte: Honoraria, Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria; Pfizer Inc.: Honoraria. Moiraghi:Pfizer: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau. Nicolini:Pfizer: Membership on an entity's Board of Directors or advisory committees; Incyte biosciences: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; KARTOS: Consultancy; Sun Pharma Ltd: Consultancy; Novartis Services, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Pagnano:Novartis: Consultancy, Honoraria; GSK: Consultancy; Wieth/Pfizer: Consultancy, Honoraria; Astellas: Consultancy, Honoraria. Žácková:Angelini Pharma: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Rea:Incyte: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. Hughes:Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Enliven: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal